In the silent battleground of petri dishes, an invisible arms race unfolds daily—one that could determine the future of modern medicine. For decades, scientists have witnessed bacteria evolving resistance to antibiotics with alarming speed, but only recently have researchers developed methods to observe this evolutionary struggle in real time. These microbial "wars" reveal not just how superbugs emerge, but potential strategies to outmaneuver them.
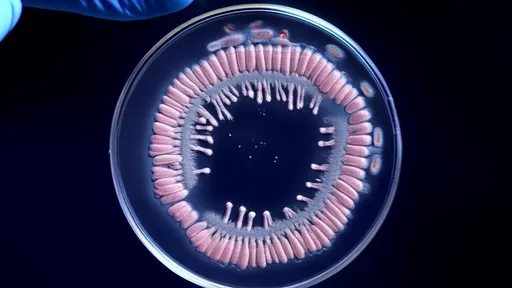
The experiment seems deceptively simple: a rectangular petri dish divided into sections containing increasing antibiotic concentrations. Scientists inoculate one end with bacteria and watch as they advance across this toxic landscape. What appears as mere cloudy streaks to the naked eye represents generations of microbial life and death—a visual chronicle of Darwinian selection pressure. Time-lapse photography captures pioneers mutating to survive lethal doses, their descendants conquering territory that would have killed their ancestors.
What makes these experiments revolutionary isn't just the visual evidence of evolution, but the predictable patterns that emerge. Bacterial colonies don't spread uniformly like spilled liquid; they advance in sudden jumps corresponding to critical genetic mutations. Researchers at Harvard Medical School observed E. coli requiring precisely four mutations to survive a 1,000-fold antibiotic increase—a genomic roadmap of resistance development. This quantifiable progression allows scientists to anticipate resistance rather than merely react to it.
Beneath the agar surface, biochemical warfare rages. Antibiotics typically target essential bacterial functions—cell wall synthesis, protein production, or DNA replication. Resistant mutants employ diverse strategies: some develop molecular pumps to eject drugs, others modify the antibiotic's target site, while a few literally eat the poison by evolving enzymes that break down the medication. The petri dish becomes an evolutionary arena where each adaptation begets new vulnerabilities.
Perhaps most surprisingly, these microbial battles demonstrate that resistance often comes at a cost. Mutations conferring antibiotic resistance frequently impair growth rates or metabolic efficiency in drug-free environments. This explains why resistant strains don't always dominate—until antibiotics enter the picture. Scientists now exploit this weakness through "collateral sensitivity," where resistance to one drug makes bacteria susceptible to another. Sequential or combination therapies could theoretically trap pathogens in an evolutionary no-win scenario.
The petri dish wars also reveal disturbing parallels to clinical realities. When researchers recreated the antibiotic concentration gradients found in human bodies during treatment, bacteria developed resistance up to 100,000 times faster than under constant exposure. This suggests that the fluctuating drug levels typical of patient dosing may inadvertently accelerate resistance—a finding that could reshape treatment protocols.
Yet these miniature evolutionary experiments offer hope alongside warnings. By preemptively mapping resistance pathways, scientists can design "evolution-proof" drugs that force bacteria into fatal genetic traps. Some teams are developing antibiotics that become more potent when bacteria mutate to resist them—essentially turning microbial evolution against itself. Others use phage therapy as precision weapons against resistant strains.
As the petri dish continues to serve as both microscope and crystal ball, one lesson becomes clear: in the war against superbugs, understanding evolution may prove more powerful than discovering new antibiotics. The bacteria will keep evolving—but now, so will our strategies to defeat them.

By /Jul 22, 2025
By /Jul 22, 2025

By /Jul 22, 2025

By /Jul 22, 2025

By /Jul 22, 2025

By /Jul 22, 2025

By /Jul 22, 2025

By /Jul 22, 2025
By /Jul 22, 2025

By /Jul 22, 2025

By /Jul 22, 2025

By /Jul 22, 2025

By /Jul 22, 2025
By /Jul 22, 2025
By /Jul 22, 2025
By /Jul 22, 2025

By /Jul 22, 2025
By /Jul 22, 2025